Alex English, March, 1999
Some time ago, list member Norbert Snef convinced me that simply measuring CO and CO2 in the flue gas was insufficient to asses combustion quality in biomass burners. Slowly over the last few months I have been making an attempt to use a device called a dilution tunnel for measuring particulate concentrations in flue gasses.
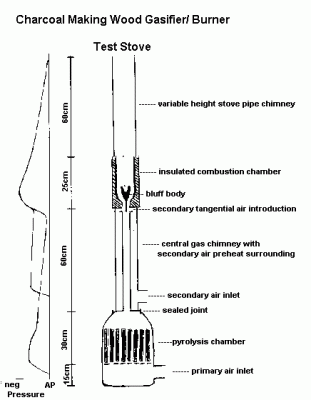
Last Saturday I found my self making some changes to the boilers at the commercial greenhouses where I work. My experiments with charcoal making burners have taken place here as well. So it was not to surprising that I saw the small burner sitting just outside the boiler room where I was working. A few weeks ago I purchased some wood pellets with the intention of trying them instead of the split cedar kindling that I usually use. So after re-jetting boiler number 1, I decided it would be a good time to try out the pellets. The pyrolysis chamber would hold four times the mass of pellets than of cedar sticks so I figured this test would take
around four hours instead of the usual one hour. I could let it burn while I worked on boiler number 2.
When this "stove" is running I can't seem to leave it alone. It
wasn't long after it was running smoothly that I decided I should
really try out the particulate sampler. (Check out "Determination of Condensible Particulate Woodstove Emission Factors Using Condar's Emissions Sampler" that Norbert has on his website.)
A previous attempt had proved to me that the gasses were to hot to be sampled. With that set up, the gasses going through the filter were over 300F. The rule of thumb is
that you need to sample with the filter temperature 60-90F. Changing the sampling and dillution rate might correct the problem. So I ran off to get the necessary stuff. Boiler #2 would have to wait.
Wood pellets are probably the ideal fuel for top down pyrolysis.
Dry, uniform small size but large enough for air to pass between.
So it wasn't to surprising that the stove was performing well.
By the time I had the particulate sampler set up, the stove was running with CO2 in the 12-14% range and CO fluctuating around 100-150ppm. At this setting the particulate emissions rate was about 0.5 g/kg of fuel consumed, depending on which set of assumptions you make about the actual composition of the fuel. (Pyrolysis gasses have a lower Carbon to Hydrogen ratio than wood.)

(Image of Filters) The filter for this test is on the bottom left. Top left filter is a new unused filter.
After that I plugged one of the secondary holes reducing excess air,
and increasing CO2 to 16-17%. CO dropped to a stable 30-40ppm. This filter is on the top right of the image of filters. There was no measurable gain in the mass of this filter. So the rate is <0.1g/kg, or darn close to zero.
Next I tried to get a sample of the smoke that is the fuel for this
fire. (The smoke that most charcoal kilns produce) Normally I can
snuff the flame by closing off the secondary air holes with my hands.
Once out,it would billow smoke until a flame or spark is introduced to
re-ignite it. Not so with this one. As soon as I took my hands away,
the burner re-lit. This suggested to me that the temperatures in the combustion
chamber were higher than during previous burns using wood, higher
than the ignition point of the fuel air mixture.
I did managed to snuff the flame by blocking all the secondary air ports.
The sampler however quickly developed increasing pressure drop across
the filter resulting in reduced sampling rate. I shut it down after
five minutes. This filter is shown on the bottom right. It is a bit of a guess as to how to interpret the mass gain of this filter as it is a sample of fuel as opposed to the normal sample of flue gas with excess air. I took a stab at it anyhow. The emissions rate would be up around 100g/kg.
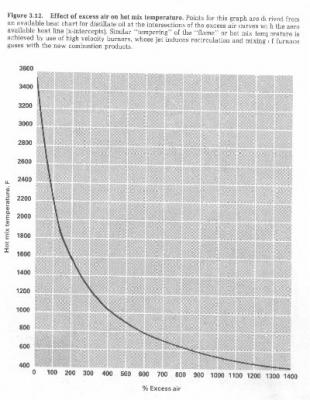
These results prompted me to back to a graph which shows the effect of excess air on "hot mix temperatures". This burner has more in common with the oil burners that I work with than it does with most wood stoves. The combusting gasses are thoroughly mixed within a conforming combustion chamber. Although the values on the verticle axis of this graph are not relevant to this fuel, the shape of the graph is. The very steep portion of the curve of < 100% excess air is the region where this stove was operating for the first two samples. The second sample was taken when excess air was even less than the first. This would result in a significant temperature rise. Filter #1 yeilded a very low emissions factor. Still some tars were present. The higher temperature conditions for filter sample #2 took care of those tars.
The burn lasted about 3.5 hours. The first few minutes and that last
few, are periods where the output is less stable. During the last few minutes, char combustion on the bottom layer coincides with a decline in production of pyrolysis products available for the combustor.
After that test I headed back to boiler #2. I struggled
with high pressure oil atomizing burner for a couple of hours and
never got it going. I went home with the irony that at least on this day,
the low tech biomass burner had out classed the high tech oil
burner that we use to heat the greenhouses.
So if the pellets contain 7500 btus/pound, the burner pyrolysed 10
pounds per hour. If the gasses contain 2/3 of the energy then the
burner put out about 50000 btus/hr. The top down pyrolysis progressed
downward at a rate of 4 inches per hour. The cross sectional area was
about 3/4 sqft. Then a barrel five feet tall and four feet in
diameter filled with pellets would be able to provide 800,000 btus/hr
for fifteen hours (over night). Three or four of them might heat this
place and produce heaps of charcoal. Hmmmm.....